Abstract
Dexamethasone (Dex) is a key component of ALL therapy, with glucocorticoid sensitivity strongly linked to prognosis. However, it also contributes to both short and long term toxicities. The UKALL 2011 trial (ISRCTN64515327) investigated a shorter schedule of Dex as part of randomisation 1 (R1) (10mg/m2 x 14 days vs 6mg/m2 x 28 days), in an attempt to bring about a more rapid cytoreduction whilst limiting toxicities associated with long term steroid exposure. There are limited data regarding Dex pharmacokinetics, however large variability has been reported in children being treated for ALL (Yang et al. 2008).
To investigate Dex pharmacokinetics in the context of the UKALL2011 randomised dex dosing and scheduling, blood samples were collected up to 8h post oral administration of Dex on one of the first three days, and one of the last three days of induction chemotherapy. Plasma Dex levels were analysed using a fully validated tandem reverse phase LC-MS method, and non-compartmental pharmacokinetic analysis was performed using Pheonix WinNonlin.
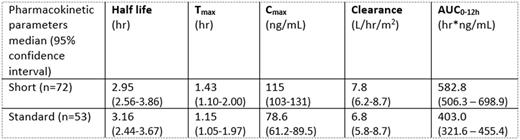
Pharmacokinetic parameters from day one sampling are shown in the table below. Exposure, as defined by AUC0-12h, and Cmax were significantly higher on the short arm (n=72) compared to the standard arm (n=53) (p=<0.0001 for both). However, there was substantial overlap between the two arms, with a number of patients on the standard arm exhibiting higher exposures than those on short therapy, despite having a longer duration of treatment, and vice versa. This variation is displayed in the greater than 20-fold variation in AUC0-12h values observed on the two arms (short: 69.1-1,606; standard: 38.3-1,009 hr*ng/mL). When AUC values were extrapolated to the duration of Dex therapy on short and standard arms, patients on the standard arm had a significantly larger overall exposure compared to those on the short arm (short: 16,318 (14,177-19,568) vs. standard: 22,570 (18,012-25,550) hr*ng/mL, median (95% CI) p=0.01).
Early response data would indicate a trend towards a higher Dex exposure (AUC) in patients with a day 8 bone marrow blast count <5% versus those patients with a day 8 blast count >5 % (595.3 (458.5-843.7) vs 441.7 (387.5-552.1) hr*ng/mL, median (95% CI) p=0.0006). However, there was no statistical difference between the two randomisation arms in terms of day 8 blast count (p=0.2). Pharmacokinetic profiles also differed between the two days of treatment, with AUC0-12h being significantly higher at the end of induction chemotherapy (n=52, study day 1: 562.5 (435.7-756.5); study day 2:794.5 (648.7-1041) hr*ng/mL, (median (95% CI) p=0.0003).
The UKALL 2011 R1 found no statistical difference in terms of steroid related toxicity or MRD response between short and standard Dex dosing. The data generated from patients recruited to the pharmacology sub-study shows considerable variation in Dex pharmacokinetics and suggest that pharmacokinetic variability may be a more important factor than variation in dosing regimen on the two arms of the randomisation. Furthermore, a significantly higher cumulative exposure of patients on the standard arm suggests that in a drug treatment with markedly variable pharmacokinetics, duration of therapy may be more important in terms of the likely impact on clinical response and toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.